



The antiepileptic drug, a pyrrolidone derivative (S-enantiomer of alpha-ethyl-2-oxo-1-pyrrolidine-acetamide), differs in its chemical structure from the known antiepileptic drugs. The mechanism of action of levetiracetam is not fully understood, but it is obvious that it differs from the mechanism of action of known antiepileptic drugs.
In vitro studies have shown that levetiracetam affects the intra-neuronal concentration of Ca ions.2+partially braking the current Sa2+ through N-type channels and reducing calcium release from intra-neuronal depots. In addition, levetiracetam partially restores ionic currents through GABA- and glycine-dependent channels reduced by zinc and carbolines.
One of the putative mechanisms is based on proven binding to the glycoprotein of the SV2A synaptic vesicle contained in the gray matter of the brain and spinal cord. It is believed that in this way the anticonvulsant effect is realized, which is expressed in counteracting the hypersynchronization of neural activity. Does not alter the normal neurotransmission, however, suppresses epileptiform neuronal outbreaks induced by the GABA agonist bicculin and the excitation of glutamate receptors. The activity of the drug is confirmed in relation to both focal and generalized epileptic seizures (epileptiform manifestations / photoparoxysmal reaction).
- additional therapy of partial seizures in adults and children over 12 years of age with epilepsy.
| 1 tab. | |
| levetiracetam | 500 mg |
Excipients: Povidone-K30 - 12 mg, Povidone-K90 - 13 mg, ethylcellulose - 100 mg, dibutyl sebacate - 15 mg, microcrystalline cellulose - 80 mg, castor oil, hydrogenated - 40 mg, magnesium stearate - 6 mg.
The composition of the film shell: Opadry 03F18435 white - 16 mg (hypromellose - 9.8 mg, macrogol-3350 - 3.26 mg, titanium dioxide - 2.94 mg);ink Opacode S-1-17823 black (pharmaceutical glaze [shellac solution in ethanol] 44.467%, isopropanol 26.882%, iron dye black oxide (E172) 23.409%, butanol 2.242%, propylene glycol 2%, aqueous ammonia 1%).
Levetiracetam is marketed under different brands and generic names, and comes in different dosage forms:
| Brand name | Manufacturer | Country | Dosage form |
|---|---|---|---|
| pills | |||
| Levetiracetam | Canonpharma | Russia | pills |
| Levetinol | Geropharm | Russia | pills |
| Keppra | UCB Pharma | Belgium | solution |
| Keppra | UCB Pharma | Belgium/Russia | pills |
No customer reviews for the moment.
Administered orally, washing down with enough liquid, irrespective of meal.
Adults and children over 12 years old: the initial dose is 1000 mg 1 time per day. The dose can be adjusted by an increase of 1000 mg every 2 weeks. The maximum daily dose is 3000 mg.
In elderly patients and patients with renal insufficiency, the dose should be adjusted depending on the CC. QC can be calculated based on the concentration of creatinine in the serum, according to the following formula.
For men:
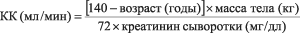
For women: multiply the resulting value by 0.85
Then, the spacecraft is corrected with regard to the body surface area (PPT) according to the following formula:
QC (ml / min / 1.73 m2) = QC (ml / min) / PPT (m2) ×1.73
| Kidney function | QC (ml / min / 1.73 m2) | Dose and frequency of administration |
| Norm | more than 80 | 1000-3000 mg every 24 hours |
| Mild degree of impairment | 50-79 | 1000-2000 mg every 24 hours |
| The average degree of violation | 30-49 | 500-1500 mg every 24 hours |
| Severely disturbed | less than 30 | 500-1000 mg every 24 hours |
In patients with hepatic insufficiency, dose adjustment is not required.
The safety profile presented below is based on an analysis of the results of clinical studies and post-registration experience.
The most common adverse reactions were nasopharyngitis, drowsiness, headache, fatigue, and dizziness. The profile of levetiracetam is generally similar for different age groups of adults and children.
Category determination of the frequency of adverse reactions: very often (≥1 / 10), often (≥1 / 100, <1/10), infrequently (≥1 / 1000, <1/100), rarely (≥1 / 10 000, < 1/1000), very rarely (<1/10 000).
Infections and invasions: very often - nasopharyngitis; rarely infections.
From the hemopoietic system: infrequently - leukopenia, thrombocytopenia; rarely - pancytopenia, neutropenia, agranulocytosis.
On the part of the immune system: rarely - drug reaction with eosinophilia and systemic manifestations (DRESS syndrome), hypersensitivity (including angioedema and anaphylactic reaction).
Metabolism: often - anorexia; infrequently - weight gain, weight loss; rarely - hyponatremia.
Mental disorder: often - depression, hostility / aggressiveness, anxiety, insomnia, nervousness / irritability; infrequently - suicidal attempts, suicidal thoughts, psychotic disorders, behavioral disorders, hallucinations, anger, confusion, emotional lability / mood swings, arousal, panic attack; rarely - suicide, personality disorder, impaired thinking.
On the part of the nervous system: very often - drowsiness, headache; often - convulsions, imbalance, dizziness, lethargy, tremor; infrequently - amnesia, memory impairment, impaired coordination / ataxia, paresthesia, decreased concentration of attention; rarely - choreoathetosis, dyskinesia, hyperkinesia.
On the part of the organ of vision: infrequently - diplopia, blurred vision.
From the organ of hearing: often - vertigo.
On the part of the respiratory system: often - cough.
On the part of the digestive system: often - abdominal pain, diarrhea, dyspepsia, vomiting, nausea; pancreatitis.
Liver and biliary tract: infrequently - changes in functional tests; rarely - liver failure, hepatitis.
On the part of the skin and subcutaneous tissues: often - skin rash; infrequently - itching, eczema, alopecia; rarely, toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme.
From the musculoskeletal system: infrequently - myalgia, muscle weakness.
Other: often - asthenia / fatigue; infrequently - accidental damage.
The risk of anorexia is higher with simultaneous use of levetiracetam and topiramate.
There are reports of hair restoration after the abolition of levetiracetam.
Some cases of pancytopenia were accompanied by inhibition of bone marrow function.
The safety profile of levetiracetam in children in placebo-controlled clinical trials was comparable to that in adults.
- hypersensitivity to levetiracetam, pyrrolidone derivatives or other components of the drug;
- children's age up to 12 years.
With care: renal failure, liver disease in the stage of decompensation, elderly patients (over 65 years).
Levetiracetam does not affect the concentration of antiepileptic drugs (phenytoin, carbamazepine, valproic acid, phenobarbital, lamotrigine, gabapentin and primidone) in the blood plasma. In turn, these antiepileptic drugs do not affect the plasma concentration of levetiracetam.
There are no data on clinically significant drug interactions in children who received levetiracetam 60 mg / kg / day.
A retrospective analysis of pharmacokinetic interactions in children aged 4 to 17 years suffering from epilepsy confirmed that supplemental ingestion of levetiracetam does not affect the equilibrium plasma concentrations of simultaneously taken carbamazepine and valproic acid. However, evidence suggests that clearance of levetiracetam is 22% higher in children taking enzyme-induced antiepileptic drugs. Dose adjustment in these cases is not required.
Levetiracetam at a daily dose of 1000 mg does not change the pharmacokinetics of oral contraceptives of ethinyl estradiol and levonorgestrel.
Levetiracetam in a daily dose of 2000 mg does not change the pharmacokinetics of warfarin and digoxin.
Digoxin, oral contraceptives and warfarin do not affect the pharmacokinetics of levetiracetam.
Probenecid (500 mg 4 times / day) reduces the renal clearance of the primary metabolite of levetiracetam. It is assumed that drugs that are excreted from the body through active renal secretion can also reduce the clearance of the metabolite. The effect of levetiracetam on probenecid and other drugs with a similar elimination mechanism (NSAIDs, sulfonamides, methotrexate, etc.) remains unclear.
No data on the effect of antacids on the absorption of levetiracetam.
There have been isolated reports of a decrease in the effectiveness of levetiracetam with simultaneous use with osmotic laxative macrogol. Macrogol should not be taken within 1 hour before and within 1 hour after taking levetiracetam.
The degree of absorption of levetiracetam does not change under the influence of food, while the rate of absorption decreases slightly.
Data on the interaction of levetiracetam with alcohol are not available.
In the post-marketing data obtained from several prospective registers of pregnancies, more than 1000 cases of using levetiracetam as monotherapy were recorded in the first trimester of pregnancy. Overall, these data do not indicate a significant increase in the risk of serious congenital malformations. Teratogenic damage cannot be completely excluded. Therapy with multiple antiepileptic drugs is associated with a higher risk of congenital malformations than monotherapy, therefore, monotherapy in pregnant women is more appropriate.
Adequate and strictly controlled clinical studies on the safety of levetiracetam in pregnant women have not been conducted, therefore Epiterra Long is used in pregnancy only if the expected benefit to the mother exceeds the potential risk to the fetus.
Physiological changes in a woman’s body during pregnancy can affect the concentration of levetiracetam, as well as other antiepileptic drugs, in the blood plasma. During pregnancy, a decrease in plasma concentration of levetiracetam was observed. This decrease was expressed in the third trimester of pregnancy (up to 60% of the baseline concentration during the third trimester). The use of levetiracetam in pregnant women should be carried out under special control. It should be borne in mind that interruptions in the conduct of antiepileptic therapy may worsen the course of the disease, which may harm the health of both the mother and the fetus.
Levetiracetam is excreted in breast milk, so the use of the drug Epiterra Long during breastfeeding is not recommended.However, if breastfeeding is required during breastfeeding, the benefit / risk ratio of the treatment should be assessed taking into account the importance of breastfeeding.
In animal studies, no effect of levetiracetam on fertility was found. Clinical data on the effect of levetiracetam on fertility are not available, the possible risk to humans is unknown.
If it is necessary to discontinue therapy, it is recommended to discontinue Epiterra Long gradually, reducing the single dose by 500 mg every 2-4 weeks. In adults with a body weight of less than 50 kg, it is recommended to reduce the dose by 20 mg / kg every 2 weeks. In children with a body weight of 25-50 kg, it is recommended to reduce the dose by 10 mg / kg every 2 weeks.
The use of the drug Epiterra Long in patients with impaired renal function may require dose adjustment (see section "Dosage regimen").
While taking the drug Epiterra Long, violations of the central nervous system can be observed, including drowsiness, weakness, fatigue, psychotic and non-psychotic behavior changes, especially during the first month of therapy, so patients should be especially carefully monitored at the beginning of treatment.
Patients should be regularly examined for signs of depression, suicidal and obsessive ideas, due to the increased risk of suicide attempts in patients taking anti-epileptic drugs, including drug Epiterra Long.
If there are signs of depression and attempted suicide, patients and their caregivers should contact a specialist.
A gradual abolition of concomitant anticonvulsants is recommended during the period when a patient is transferred to the drug Epiterra Long.
In patients with partial seizures, a slight but statistically significant decrease in red blood indices was observed during treatment with the drug, which does not require active clinical intervention.
Use in Pediatrics
Available information about the use of the drug in children does not indicate any of its negative effects on development and puberty. However, the long-term effects of treatment on children's ability to learn, their intellectual development, growth, endocrine gland functions, sexual development and fertility remain unknown.
Influence on ability to drive vehicles and mechanisms
No studies have been conducted on the effect on the ability to drive vehicles and work with mechanisms. Due to the possible different individual sensitivities, some patients may experience drowsiness or other symptoms from the CNS, especially at the beginning of treatment or after a dose increase. Thus, such patients need to be careful when performing tasks such as, for example, driving a vehicle or mechanism.
Patients are advised not to drive the vehicle and not to use the mechanisms until the absence of an influence on the ability to potentially dangerous activities is established.
Symptoms: drowsiness, agitation, aggression, depression of consciousness, respiratory depression, coma.
Treatment: in the acute period - induction of vomiting, gastric lavage, taking activated charcoal. There is no specific antidote for levetiracetam. If necessary, symptomatic treatment is carried out in the hospital with hemodialysis (dialysis for levetiracetam is 60%, for the primary metabolite 74%).
Studies and clinical trials of Levetiracetam (Click to expand)